Talk:Cyclohexane
| This It is of interest to the following WikiProjects: | |||||||||||||||||||||
| |||||||||||||||||||||
Untitled
[edit]Comment from 216.185.236.2 moved from page text: gnjshgisjgoSKJGosKfgp\ —Preceding unsigned comment added by 60.51.209.164 (talk) 06:27, 19 March 2008 (UTC)
Note: Somebody with more ambition than myself really ought to post some pics of this stuff. A picture is worth a 1000 words eh? :)
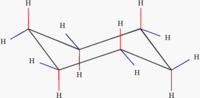
Which of the following structures is correct?
Thank you! User:Sverdrup 21:14, 29 Jun 2004 (UTC)
- They are all the same. Only the last shows a 3-D view of the structure, the first two are only symbolic. And please don't use images in your Wiki signature, it is rude. -- Tarquin 21:20, 29 Jun 2004 (UTC)
Ok, I just wanted to know which one was preferred, but nr 2 is good in the article. I don't know if it's rude to have a pic in the signature, but maybe it will bloat the talk pages if the trend catches. Anyhow, I added an image to my signature to get a debate about it, and it seems to have started now :-) ✏ Sverdrup 21:24, 29 Jun 2004 (UTC)
All three are correct, however, the third is the most informative, as it graphically shows a typical cyclohexane conformation (which should be noted in the article or the image's caption. -- Seth Ilys 21:45, 29 Jun 2004 (UTC)
- Thank you both for the input. I colored the third one quite crudely, but I think it has some educational value. ✏ Sverdrup 22:02, 29 Jun 2004 (UTC)
Contradicting Molar masses
[edit]The molar masses in the article and the side info bar don't match, 84.16 g/mol is correct.
I agree. The molar mass of cyclohexane is 84.1608 g/gmol (12.011*6 + 1.0079 *12 = 84.1608). I updated the side info bar to reflect this. Dxmnkd316 (talk) 20:26, 24 February 2009 (UTC)
3D Model
[edit]Why are the hydrogens so huge? Isopropyl 18:39, 27 May 2006 (UTC)
- They look about the right size to me. Remember, although hydrogen has 1/12 the mass of carbon, it's the electrons in their atomic orbitals that determine the effective radius. The largest filled orbital of H is 1s, and the largest filled orbital of C is 2p, which is about twice as large I think, depending on the probability threshold you use. —Keenan Pepper 21:02, 27 May 2006 (UTC)
"The usefulness of cyclohexane in research is not to be underestimated."
Hahaha, most useless sentence evah!
--- —Preceding unsigned comment added by 67.70.99.163 (talk) 02:29, 7 October 2007 (UTC)
- You're right. I removed it. --Ed (Edgar181) 13:05, 7 October 2007 (UTC)
In Popular Culture...
[edit]Cyclohexane makes an appearance in the movie "War" when Agent Crawford goes back to meet Rogue at the docks. (1h 32m 20s into the movie).
Just saying. :D DEMONIIIK (talk) 06:34, 14 August 2008 (UTC)
Cyclohexane is also featured in the 1950s Dragnet episode "The Big Lamp" as supposedly being prominently visible in ultraviolet light. No mention of this property in the article, but Jack Webb had a reputation for insisting on technical accuracy in his projects. 99.40.198.205 (talk) 20:33, 8 March 2013 (UTC)
- No, cyclohexane is not unusually visible in ultraviolet light. — Preceding unsigned comment added by 148.177.1.210 (talk) 21:13, 8 March 2013 (UTC)
Production and use
[edit]Here in wikipedia, on the site about benzene, we can read:"Benzene and derivatives convert to cyclohexane and derivatives when treated with hydrogen at 450 K and 10 atm of pressure with a finely divided nickel catalyst".
The article also doesn't tells if cyclohexane is used as an adictive to gasoline. Agre22 (talk) 20:22, 2 November 2009 (UTC)agre22
Baeyer synthesis
[edit]The treatment of cyclohexanol with hydrogeniodide is unlikely, even in the hands of von Bayer, to have yielded more than trace amounts of methylcyclohexane. A more likely product is cyclohexanone. The file: CyclohexaneBayerSynth.png should be corrected. — Preceding unsigned comment added by 83.93.187.21 (talk) 17:43, 1 November 2012 (UTC)
- The source for the image is citation no 3. V8rik (talk) 21:27, 1 November 2012 (UTC)
That's not methylcyclohexane, that's iodocyclohexane.--Rifleman 82 (talk) 01:08, 2 November 2012 (UTC)
External links modified
[edit]Hello fellow Wikipedians,
I have just modified 2 external links on Cyclohexane. Please take a moment to review my edit. If you have any questions, or need the bot to ignore the links, or the page altogether, please visit this simple FaQ for additional information. I made the following changes:
- Added archive https://web.archive.org/web/20100707214900/http://www.chem.yale.edu/~chem125/125/history/Baeyer/Sachse.html to http://www.chem.yale.edu/~chem125/125/history/Baeyer/Sachse.html
- Added archive https://web.archive.org/web/20060409110115/http://www.labnews.co.uk/new_labnews/article.php?artid=1017&categoryid=2&scheme=2 to http://www.labnews.co.uk/new_labnews/article.php?artid=1017&categoryid=2&scheme=2
When you have finished reviewing my changes, you may follow the instructions on the template below to fix any issues with the URLs.
This message was posted before February 2018. After February 2018, "External links modified" talk page sections are no longer generated or monitored by InternetArchiveBot. No special action is required regarding these talk page notices, other than regular verification using the archive tool instructions below. Editors have permission to delete these "External links modified" talk page sections if they want to de-clutter talk pages, but see the RfC before doing mass systematic removals. This message is updated dynamically through the template {{source check}} (last update: 5 June 2024).
- If you have discovered URLs which were erroneously considered dead by the bot, you can report them with this tool.
- If you found an error with any archives or the URLs themselves, you can fix them with this tool.
Cheers.—InternetArchiveBot (Report bug) 00:19, 16 August 2017 (UTC)
No information about safety
[edit]It would be good if there were a section about the effects of this chemical on human health. 137.111.13.43 (talk) 01:15, 14 February 2023 (UTC)
- not much to say, it is equivalent to gasoline (petrol). Nothing specific. --Smokefoot (talk) 02:08, 14 February 2023 (UTC)
- B-Class vital articles
- Wikipedia level-5 vital articles
- Wikipedia vital articles in Physical sciences
- B-Class level-5 vital articles
- Wikipedia level-5 vital articles in Physical sciences
- B-Class vital articles in Physical sciences
- B-Class Occupational Safety and Health articles
- Unknown-importance Occupational Safety and Health articles
- WikiProject Occupational Safety and Health articles
- B-Class chemicals articles
- Mid-importance chemicals articles